
Year 7 Science Lesson Solute, Solvent, Solution EdPlace YouTube
The solutions can be classified based on the physical state of the solution, solvent, and solute. For example, the air is gas in a gas solution; carbonated water is a gas in a liquid solution; vinegar is a liquid in a liquid solution; metal alloys are solid in solid solutions. Table 5.1.1 lists the major types of solutions, solvents, and.
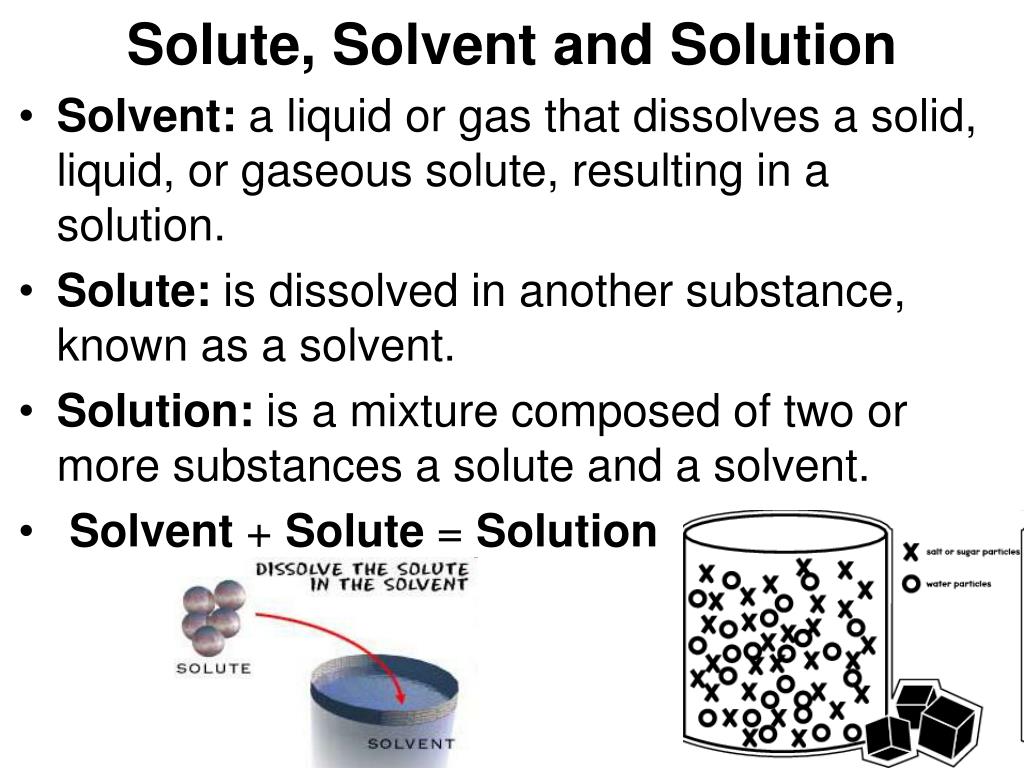
PPT Revision PowerPoint Presentation, free download ID5491285
The solutions that are exemplified in Exercise 7.2.1 7.2. 1 contain solid, liquid, and gaseous solutes and solvents. As shown below in Table 7.2.1 7.2. 1, solutions can be prepared using solvents and solutes in any state-of-matter combination, and the physical form of the resultant solution corresponds to the phase of its constituent solvent.

Solute vs Solvent Biology lessons, Solvent, Science education
Cañada College CHEM 210: General Chemistry I, An "Atoms Up" Approach 11: Solutions, Concentration, and Dilution 11.1: Solutes and Solvents
Chemistry Solutions And Mixtures Level 2 activity for kids PrimaryLeap.co.uk
solute: The component of a solution in the smallest amount. solution: Mixture made of two or more substances. solvent: The component of a solution in the largest amount. Assessment Pre-Activity Assessment. Predictions: Have students write down predictions for what they think will happen during the activity. Have them identify the components of.

Difference between solute and solvent YouTube
3.4K 242K views 6 years ago New AP & General Chemistry Video Playlist This chemistry video provides a basic introduction into solubility and how compounds dissolve in water. It discusses how water.
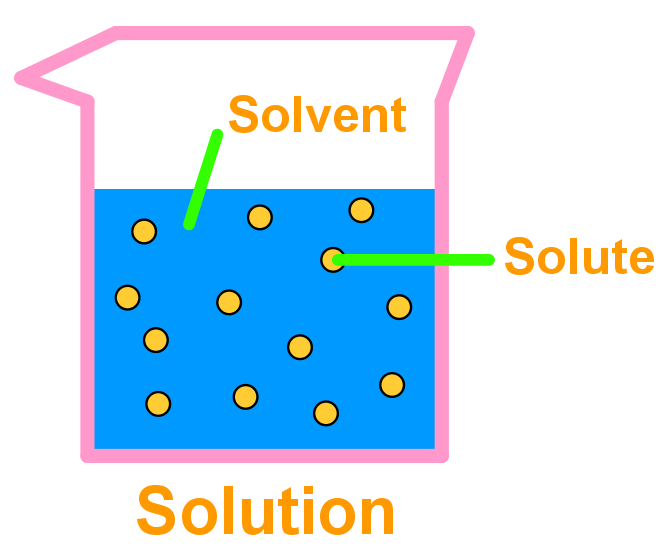
Introduction to solution chemistry and solubility StudyPug
What is a Solute? Solvent vs Solute with Examples Solute and solvent are words that often go together in chemistry. When they are mixed, they make a solution. Learn about how to identify the solute and the solvent, properties of each, and real-world examples of solvents and solutes.

Difference Between Solvent and Solute Definition, Properties, Examples
Solutions Solute vs. Solvent Lesson Summary Solutions Solutions are extremely important in human life, as most body processes depend on the movement of solutions throughout the body..

Solution with examples Identify the solute and solvent YouTube
"Solution = Solutes + Solvent" Calculate the amount of 1 M NaOH aqueous solution needed to make 100 mL of 0.5 M NaOH aqueous solution.

Solute and Solvent Combinations — Overview & Examples Expii
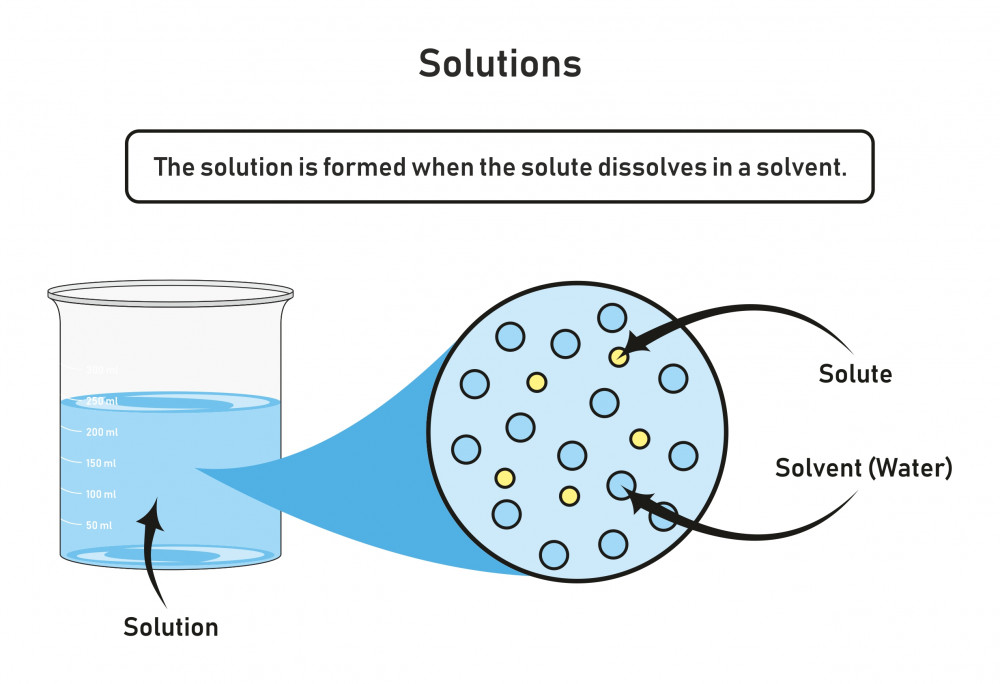
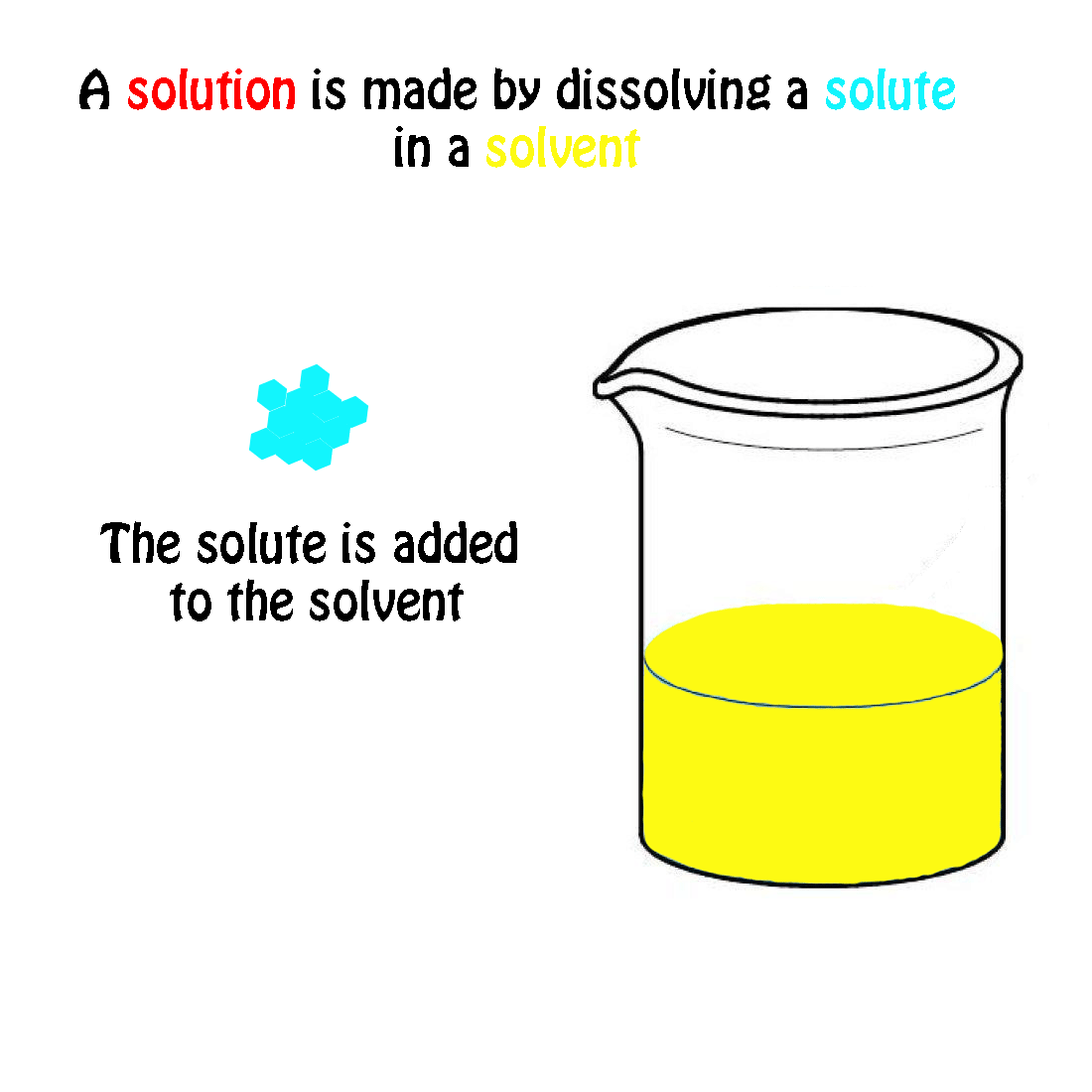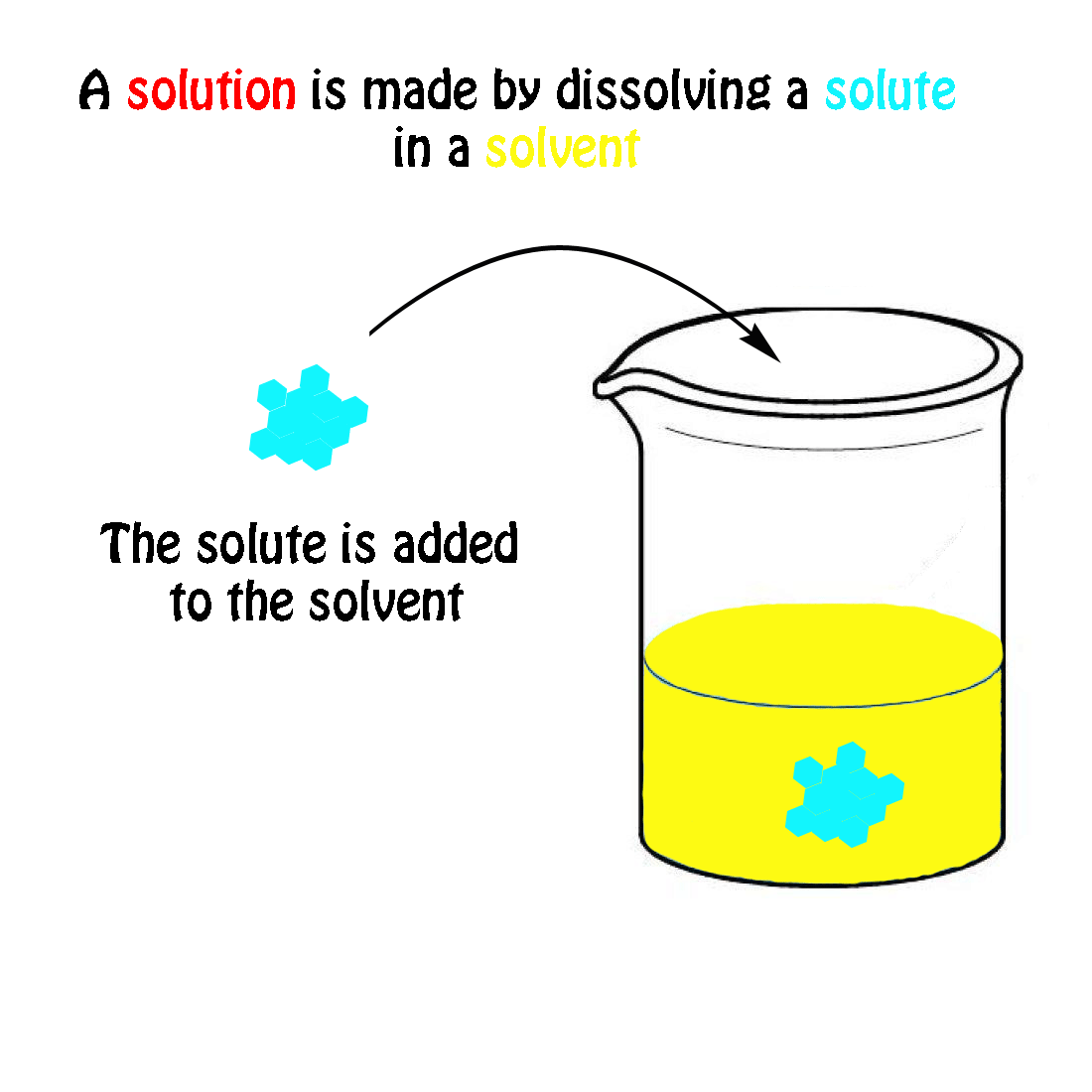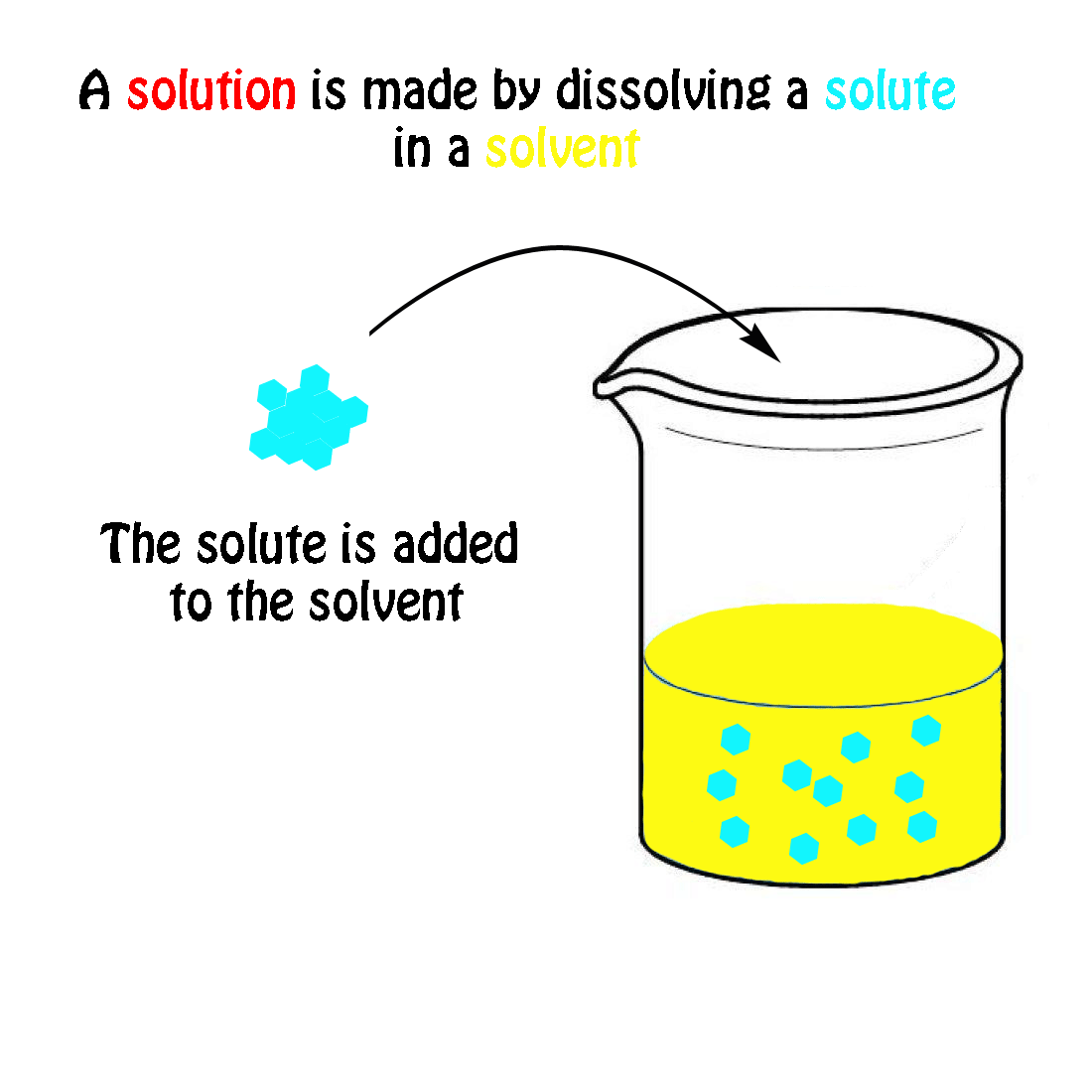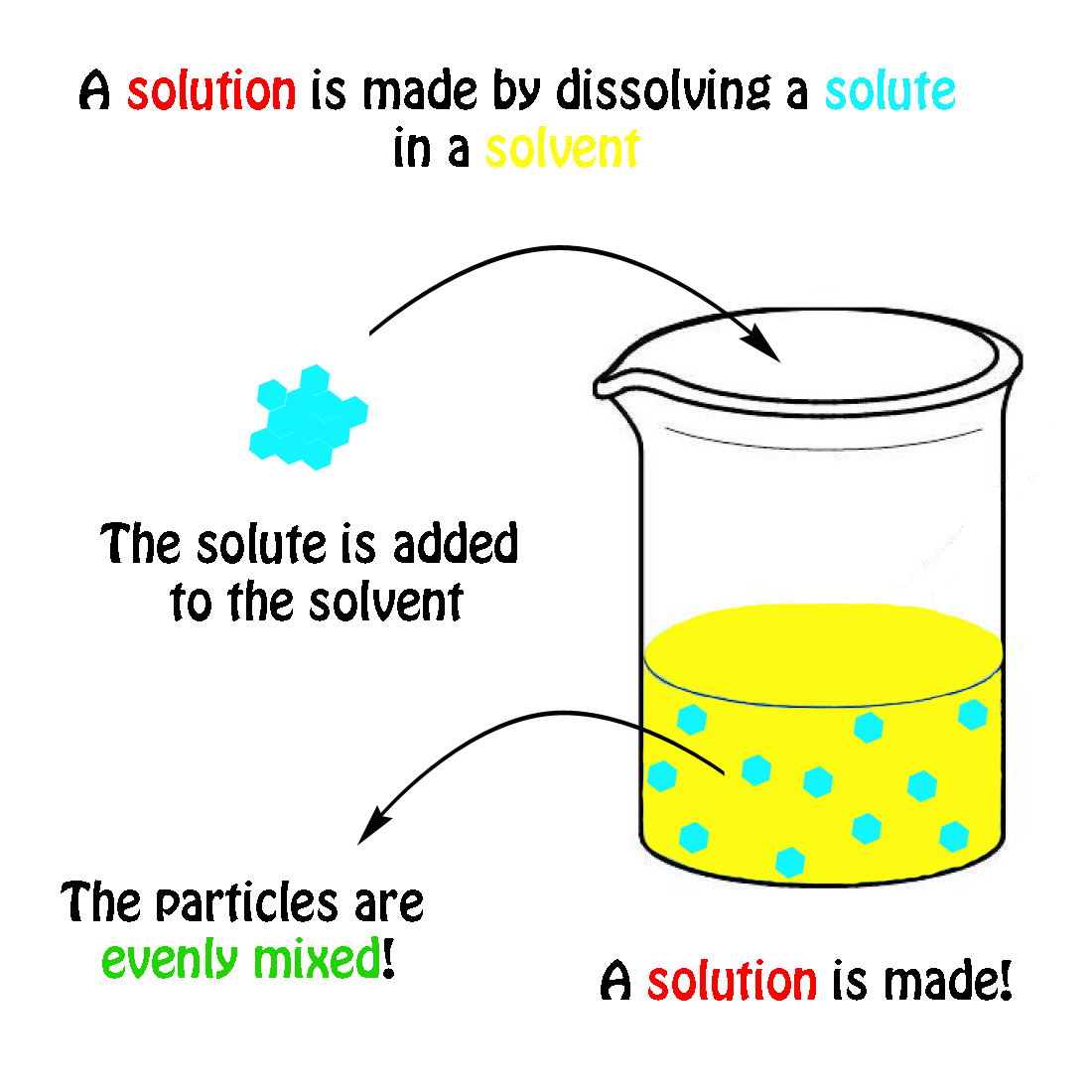
A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. The solute is the substance that is being dissolved, while the solvent is the dissolving medium. Solutions can be formed with many different types and forms of solutes and solvents. We know of many types of solutions. Check out a few examples in the table below.
5 Significant Difference between Solute and Solvent Core Differences
. If a substance can dissolve into a solvent, it is soluble . If it cannot dissolve, it is described as insoluble. Heating, stirring and using fine powders are all ways to speed up dissolving..
Solution Definition, Types, Properties Chemistry Teachoo
Solute, Solvent and Solution. The difference between solute and solvent can be determined based on their definition, physical appearance (basically a state of matter:- solid, liquid, or gas form), solubility, and boiling point.Let us see how! Firstly have a look at the definition of physical state, solubility and boiling point for better understanding.

Solutes, Solvents and Solutions REMOTE 1 YouTube
The solvent, or material that dissolves the solute, separates the molecules of the solute and distributes them evenly. What is Solvent? The part of a solution that is present in the greatest amount is called a solvent. It's the liquid that the solute is dissolved in. A solvent is usually a liquid.

PPT Solvent, Solute, Solutions and Solubility PowerPoint Presentation ID1821349
A solvent is simply a substance that can dissolve other molecules and compounds, which are known as solutes. A homogeneous mixture of solvent and solute is called a solution, and much of life's chemistry takes place in aqueous solutions, or solutions with water as the solvent.

Solute, Solvent and Solution Chemistry YouTube
A solution is a homogeneous mixture consisting of a solute dissolved into a solvent . The solute is the substance that is being dissolved, while the solvent is the dissolving medium. Solutions can be formed with many different types and forms of solutes and solvents. We know of many types of solutions. Check out a few examples in the Table below.

What Is the Difference Between Solute And Solvent? by Diksha Bhardwaj Sep, 2020 Medium
A solution is a homogeneous mixture of solutes, dissolved in a solvent. A homogeneous solution is a mixture of two or more components that have a uniform appearance and composition. Examples of solutions

Solute and Solvent Combinations — Overview & Examples Expii
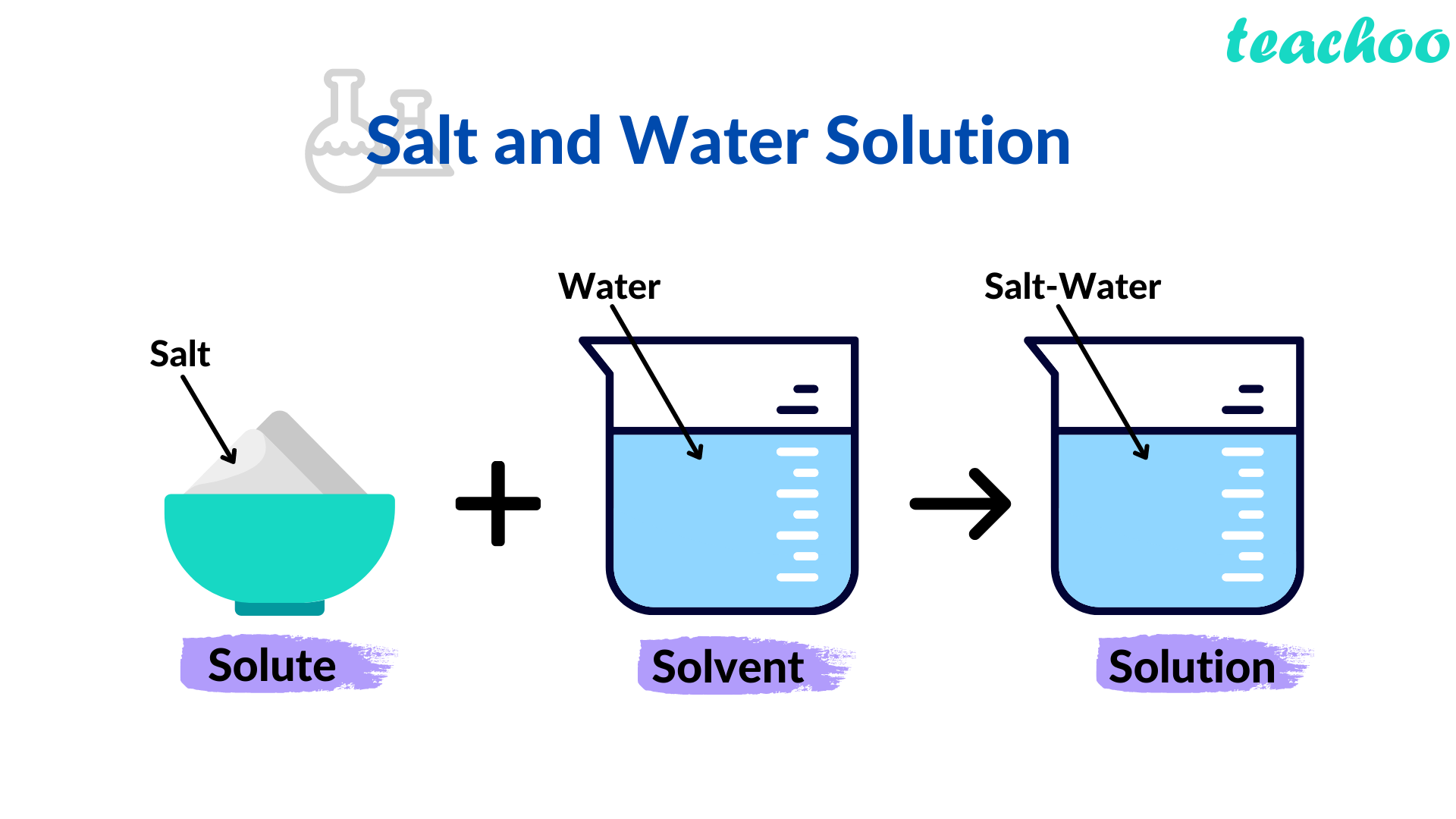
The solute is the substance in the solution that gets dissolved. The amount of solvent becomes more and greater than the Solute in the Solution. One of the easiest examples for better understanding is Salt and Water, in which salt is easily dissolved in water. Solute has the potential to take various forms like solid, liquid, or gaseous.